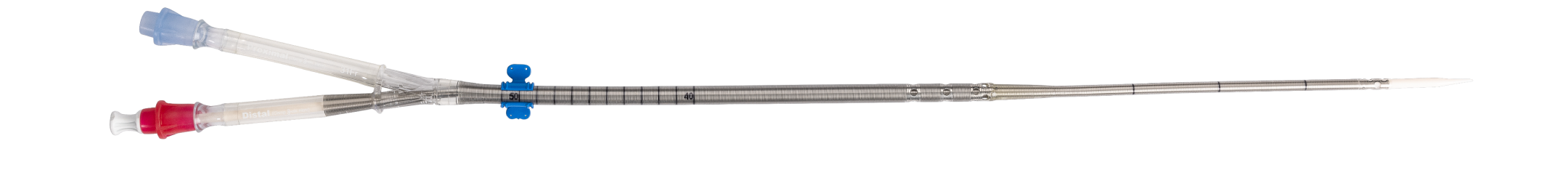

Designed to help visually differentiate the arterial and venous lumens during insertion.
Intended to make it easier to read while there is blood in the cannula.


Intended to reduce the patient’s internal exposure to the ink on the cannula body.
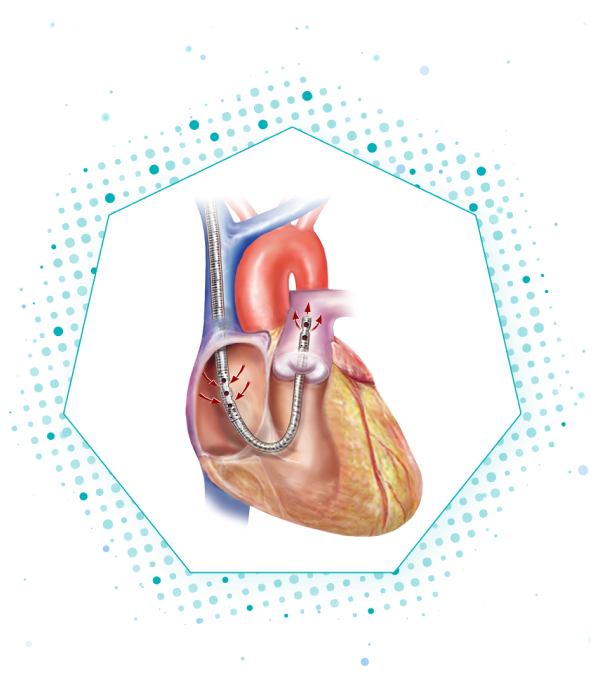
The ProtekDuo cannula pulls deoxygenated blood from the Right Atrium and returns it to the Pulmonary Artery to be oxygenated by the lungs bypassing the right ventricle.
When used with an oxygenator, the unique RA-PA configuration is designed to minimize the risk of recirculation due to the distance between the ports.
The ProtekDuo cannula has a unique cannula-within-a-cannula design that allows for percutaneous, single venous access at the Right Internal Jugular Vein.
ProtekDuo RIJ Access Provides:
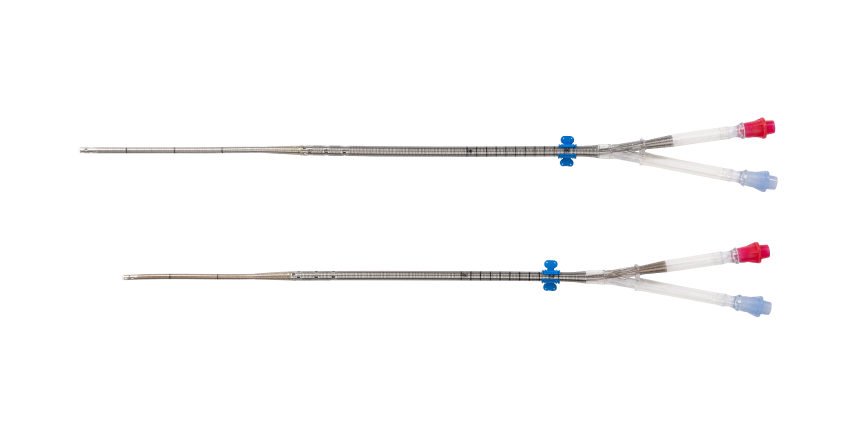
PROTEKDUO+ DUAL-LUMEN CANNULA
Technical Features
| Sizes | 31 Fr Cannula: Proximal Lumen 31 Fr x 28 cm Distal Lumen 18.5 Fr x 51 cm 29 Fr Cannula: Proximal Lumen 29 Fr x 28 cm Distal Lumen 16 Fr x 46 cm |
|---|---|
| Packaged with | One dual lumen veno-venous cannula, one introducer |
| Usage | Single Use Only |
| Sterilization | Ethylene Oxide Gas |
| Latex | Not manufactured with rubber latex |
| Insertion | Sold separately, the insertion kit includes one flow-directed PA catheter and one COOK® .035 Lunderquist® guidewire to enable smooth insertion of the ProtekDuo. |
To ensure a smooth transition, update your order system with the ProtekDuo+ product numbers.
| Size | New Product Numbers | Old Product Numbers |
|---|---|---|
| 31 Fr | 5150-5131 | 5140-5131 |
| 29 Fr | 5150-4629 | 5140-4629 |
Product guides available for download, or email to a colleague
Complete the form to stay in touch or get a call back from a LivaNova representative.
Learn more about our diverse range of specialty cannulae, including the
ProtekSolo transseptal cannula, available only from LivaNova Cardiopulmonary.
Brief Summary of Safety Information for the ProtekDuo™ Cannula Set
1. INDICATIONS FOR USE/INTENDED USE
ECMO:
The ProtekDuo+ Veno-Venous Cannula is a single use dual lumen cannula, which provides both venous drainage and reinfusion of blood via the jugular vein, that is indicated for use in adult patients with acute respiratory failure requiring Veno-Venous Extracorporeal Membrane Oxygenation, where other available treatment options have failed and continued clinical deterioration is expected or the risk of death is imminent.
CPB:
The ProtekDuo+ Veno-Venous Cannula Set is intended for use as a single cannula for both venous drainage and reinfusion of blood via an internal jugular vein during extracorporeal life support procedures.
2. Contraindications for Use
ECMO:
There are no known contraindications for the use of the cannula, other than those generally contraindicated for veno-venous (VV) ECMO. The cannula Introducer is only to be used with the appropriately sized ProtekDuo+ Veno-Venous Cannula. These devices are not intended for use except as indicated above.
CPB:
Alone, the cannula and introducer are not medical treatment devices. There are no known contraindications for the use of the cannula, other than those generally contraindicated for cardiopulmonary bypass. The cannula Introducer is only to be used with the appropriately sized ProtekDuo+ Veno-Venous Cannula. These devices are not intended for use except as indicated above.
3. Warnings & Precautions
ECMO/CPB:
Only medical personnel proficient in central venous cannulation techniques and extracorporeal life support should use this device. Follow standard percutaneous or surgical techniques to place the device. Standard medical practice for cannula insertion site care should be followed to reduce incidence of infection. The device must be used in accordance with the instructions for use.
ECMO Only:
Use of the ProtekDuo+ Cannula beyond the duration established by the available in vitro and in vivo testing has not been demonstrated. Please refer to the Instructions for Use for a summary of bench studies performed and the possible clinical observations that may necessitate or predict the need for device replacement/ change-out throughout the duration of use for this device.
For a complete listing of warnings and precautions, please refer to the Instructions for Use which accompany each product.
4. Adverse Events
ECMO/CPB:
Potential adverse events that may be associated with venous cannulation include: injury to or perforation of the myocardial wall with or without cardiac tamponade; thrombus formation; particulate or air embolism; myocardial infarction; pulmonary embolism; cardiac arrhythmias such as atrial fibrillation, heart block, sinus bradycardia, and ventricular tachycardia or fibrillation; congestive heart failure or pulmonary edema; atrial/ventricular septal defect, transient or persistent, with or without hemodynamic compromise; vascular injury with or without the need for surgical intervention; blood loss requiring fluid replacement or transfusion of blood products; infection; allergy or anaphylactic reaction to contrast media or device components; respiratory arrest; renal failure; death; failure to traverse the vascular system; hemolysis; pneumothorax (in ECMO); neurological dysfunction; ischemic stroke; Superior Vena Cava Syndrome: characterized by cyanosis, edema, and neurologic sequelae, resulting from venous occlusion.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin Group Italia S.r.l. | Via Statale 12 Nord, 86 – 41037 Mirandola (MO) Italy | Tel: +39 0535 29811
Distributed in the US by:
LivaNova USA Inc. | 14401 West 65th Way, Arvada CO 80004, United States